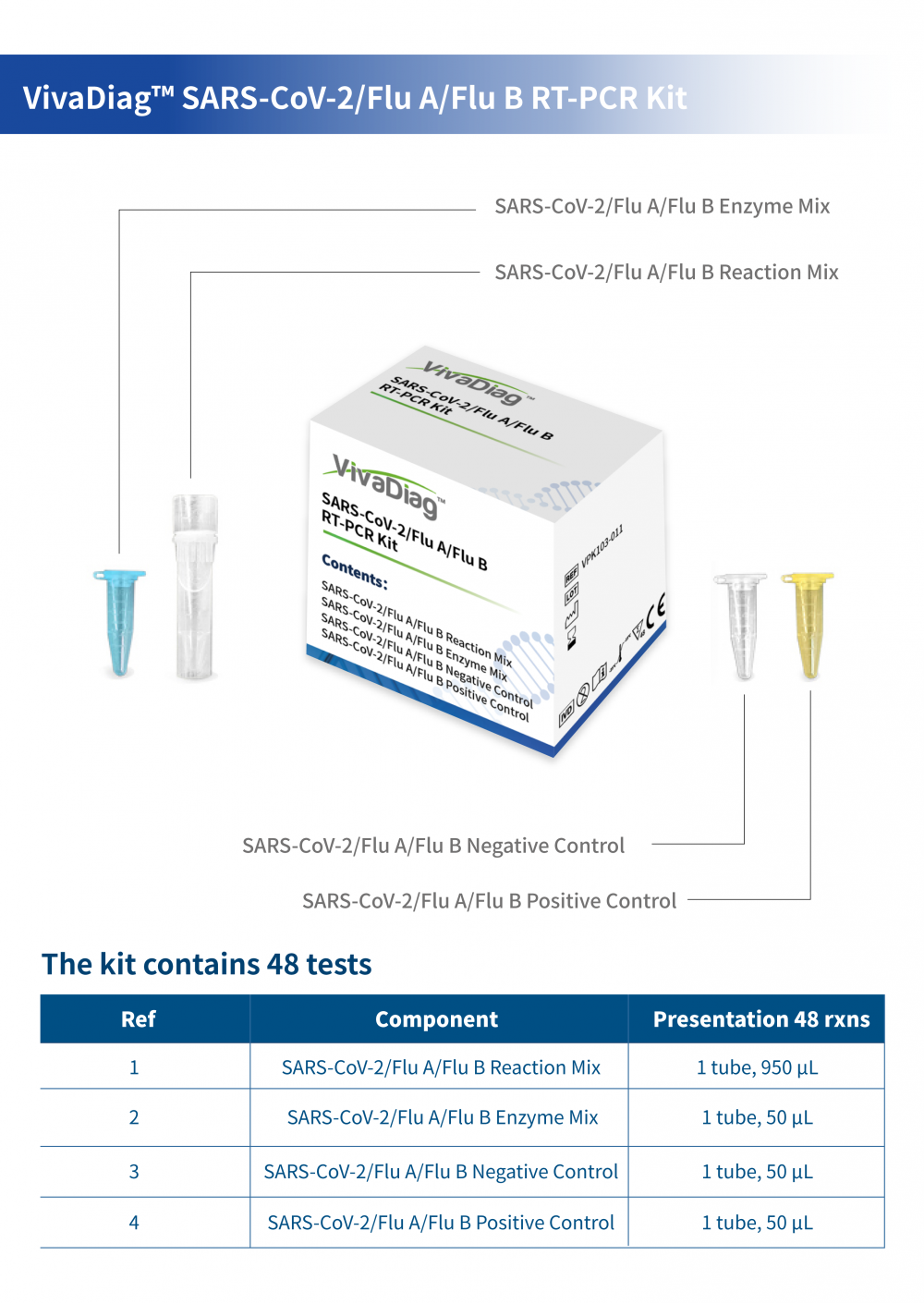
VivaDiag SARS-CoV-2/Flu A/Flu B RT-PCR Kit
- FOB Price:Get Latest Price >
- Min.Order:20 Vial(s)
- Payment Terms:EXW
- Favorite
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


VivaChek Biotech (Hangzhou) Co., Ltd.
More than 30 years of combined key leadership and experiences in the IVD industry
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

Product Introduction:
The VivaDiagTM SARS-CoV-2/Flu A/Flu B RT-PCR Kit is a multiplex, in vitro real-time RT-PCR diagnostic test intended for simultaneous qualitative detection and differentiation of SARS-CoV-2, Influenza A virus and Influenza B virus RNA from nasopharyngeal (NP) , nasal swab specimens or BALF collected in transport medium by a healthcare provider (HCP) from individuals suspected of respiratory viral infection consistent with COVID-19. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2, influenza can be similar.
Results are for the identification and differentiation of RNA from SARS-CoV-2, Influenza A and Influenza B in humans. RNA from SARS-CoV-2, Influenza A and Influenza B is generally detectable in nasopharyngeal and nasal swab specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2, Influenza A and Influenza B RNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status.